TP Straka/Leibold Central representation of locomotor correlates at the neuronal population level
PhD student Michael Forsthofer:
"Rhythmic, stereotyped locomotion such as swimming in fish/amphibians, flying inbirds or running in tetrapods is generated by central pattern generator circuitries in the spinal cord (Dietz, 1992; Orlovsky et al. 1999; Combes et al., 2004; Grillner 2006). Copies of these locomotor commands are forwarded as predictive signals to multiple supra-spinal levels for integration with sensory feedback signals and/or for the coupling of other, associated motor programs (Chagnaud et al., 2012; Straka et al., 2018). Well-studied examples for the role of such locomotor efference copies (Von Holst and Mittelstedt, 1950) or corollary discharges (Sperry, 1950) are their integration with e.g. exafferent electrosensory inputs in weakly electric fish (Requarth and Sawtell, 2014) or with motion-derived vestibular inputs in Xenopus tadpoles and adults (Lambert et al., 2012). There, these predictive signals play an important role in dissociating self-motion from passive motion and in generating an internal spatial reference frame, based on motor activity (Straka et al., 2018).
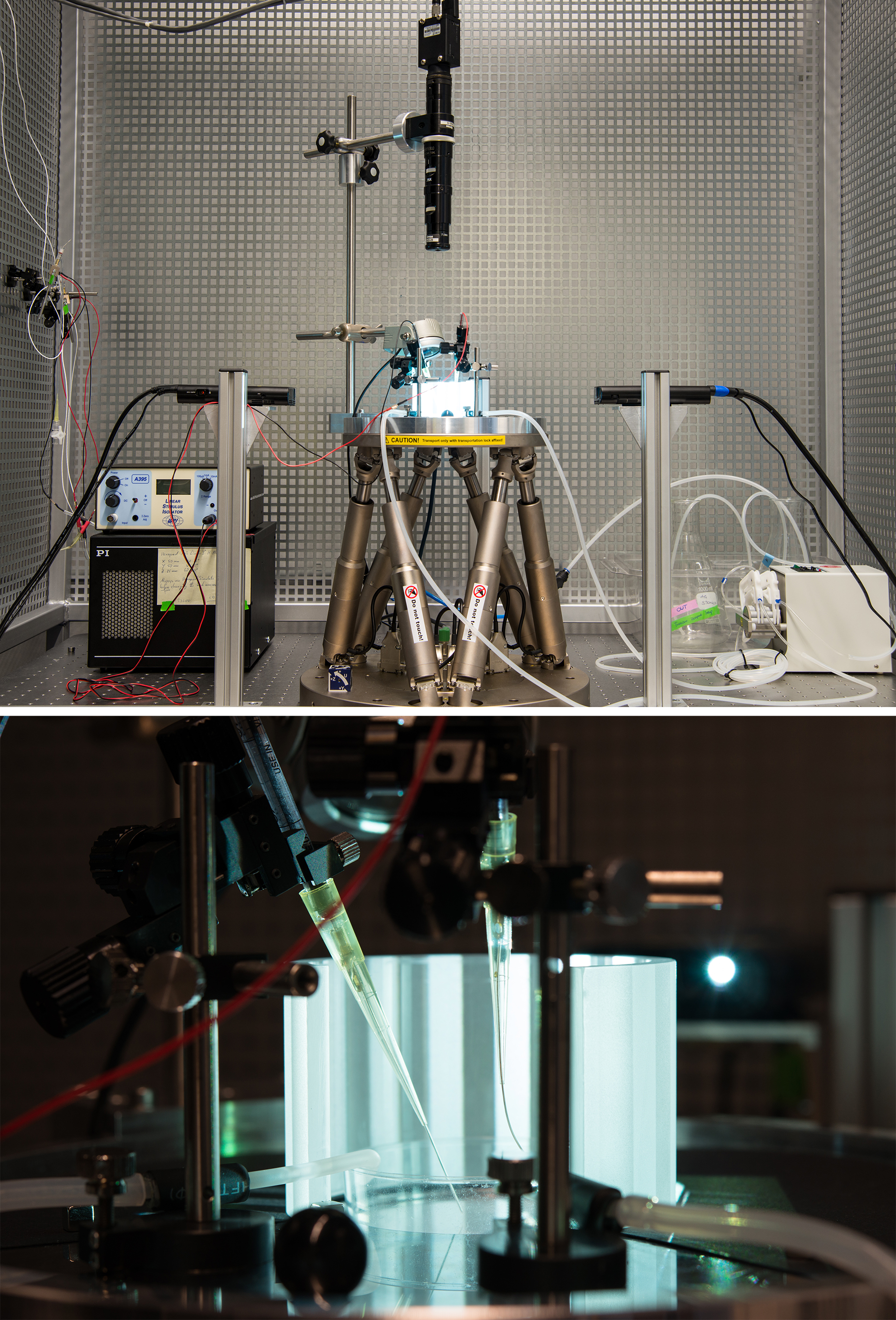
The project aims at identifying population responses at various levels of the central nervous system during locomotor activity. Particularly suitable models to conduct these experiments are isolated preparations of larval anurans (Xenopus laevis) and the salamander Axolotl (Ambystoma mexicanum). The advantage of these preparations is the maintenance of intact sensory organs (e.g. eyes, ears) in vitro for natural stimulation and the capability to record locomotor activity as neural discharge from spinal motor nerves (Straka and Simmers, 2012). The known production of spinal locomotor efference copies during fictive swimming (Lambert et al., 2012) will therefore be used to identify associated neuronal activity of cellular populations in the 1) hindbrain (vestibular/reticular nuclei), 2) cerebellum and 3) forebrain. The neuronal activity will be recorded as calcium-transients simultaneously from multiple cells following labeling of the different cell populations with calcium indicators as successfully performed in larval Axolotl (Direnberger et al., 2015) or Xenopus preparations (Gensberger et al., 2016). Sensory stimulation will be employed in a virtual reality setup with a 6d-motion simulator (Hexapod) and a whole-field visual environment (Knorr et al., 2018) that allows both combining passive visuo-vestibular activation during locomotor activity and, as part of the methods to be developed in this project, closed-loop feedback via the stimulation actuators using decoded motor outputs from fictive swimming, extraocular motor discharge or eye movements.
The experimental design allows dissecting sensory-motor from internally generated contributions to neuronal responses at the three indicated brain areas. To directly distinguish between different hypotheses, we will perform a further set of experiments in which sensory and/or motor brain regions are pharmacologically or electrically inactivated.
This allows assessing the persistence of internally generated activity patterns and possible predictions of locomotion and eye movements. Similarly, disabling activity in higher brain areas while recording from sensory regions will allow investigating the role of feedback circuits. To generate meaningful interpretations of the recordings, the project requires a high level of expertise in the analysis of multi-dimensional neuronal activity (calcium-transients, spike discharge), specifically employing methods of probabilistic decoding of motor outputs and visual stimulation, as well as the statistical quantification of such."
References
- Chagnaud BP, Simmers J, Straka H (2012) Predictability of visual perturbation during
locomotion: implications for corrective efference copy signaling. Biol Cybern 106: 669-679. - Combes D, Merrywest SD, Simmers J, Sillar KT (2004) Developmental segregation of spinal networks driving axial- and hindlimb- based locomotion in metamorphosing Xenopus laevis. J Physiol 559: 17–24.
- Dietz V (1992) Human neuronal control of automatic functional movements: interaction
between central programs and afferent input. Physiol Rev 72: 33–69. - Direnberger S, Banchi R, Brosel S, Seebacher C, Laimgruber S, Uhl R, Felmy F, Straka H, Kunz L (2015) Analysis of signal processing in vestibular circuits with a novel light-emitting
diodes-based fluorescence microscope. Eur J Neurosci 41: 1332-1344. - Gensberger KD, Kaufmann AK, Dietrich H, Branoner F, Banchi R, Chagnaud BP, Straka H (2016) Galvanic vestibular stimulation: cellular substrates and response patterns of
neurons in the vestibulo-ocular network. J Neurosci 36: 9097-9110. - Grillner S (2006) Biological pattern generation: the cellular and computational logic of
networks in motion. Neuron 52: 751–766. - Knorr AG, Gravot CM, Gordy C, Glasauer S, Straka H (2018) I spy with my little eye: a
simple behavioral assay to test color sensitivity on digital displays. Biol. Open 7: bio035725. - Lambert FM, Combes D, Simmers J, Straka H (2012) Gaze stabilization by efference copy
signaling without sensory feedback during vertebrate locomotion. Curr Biol 22: 1649-1658. - Orlovsky GN, Deliagina TG, Grillner S (1999) Neuronal control of locomotion: from mollusc to man. Oxford University Press, New York.
- Requarth T, Sawtell NB (2014) Plastic corollary discharge predicts sensory consequences of movements in a cerebellum-like circuit. Neuron 82: 896–907.
- Sperry R (1950) Neural basis of the spontaneous optokinetic response produced by visual
inversion. J Comp Physiol Psychol 43: 482–489. - von Holst E, Mittelstaedt H (1950) Das Reafferenzprinzip. Naturwissenschaften 37: 464–476.
- Straka H, Simmers J (2012) Xenopus laevis: an ideal experimental model for studying the developmental dynamics of neural assembly and sensory motor computations. Dev
Neurobiol 72: 649-663. - Straka H, Simmers J, Chagnaud BP (2018) A new perspective on predictive motor signaling. Curr Biol 28: R232-R243.
